Multidrug-Resistant Acinetobacter Infection
The genus Acinetobacter comprises glucose non-fermentative, Gram-negative, rod-shaped bacteria that cause opportunistic infections. Acinetobacter species can cause pneumonia and other respiratory tract infections, urinary tract infections, infections at sites of surgery or trauma, catheter-related bloodstream infections, sepsis, and other conditions in immunocompromised patients and others.
Under the Infectious Diseases Control Law, disease due to multidrug-resistant Acinetobacter (MDRA) infection has been designated as a Category V infectious disease under sentinel surveillance since February 2011 and as a Category V infectious disease under notifiable disease surveillance since September 2014. The Infectious Diseases Control Law defines MDRA infection as that caused by Acinetobacter species that demonstrates resistance to three types of antimicrobials: broad-spectrum β-lactam agents, aminoglycosides, and fluoroquinolones. Laboratory criteria required for notification of an MDRA infection include resistance to three types of drugs: carbapenems (e.g., imipenem), aminoglycosides (amikacin), and fluoroquinolones (e.g., ciprofloxacin). Only symptomatic patients are subject to notification of MDRA infection and asymptomatic carriers are not subject to notification (for notification criteria, see https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-05-140912-4.html). In Japan, strains that meet the laboratory criteria for notification are often defined as MDRA species.
Molecular Epidemiology of Multidrug-Resistant Acinetobacter Species
The clinically important species of the Acinetobacter genus is Acinetobacter baumannii, and the international clone lineages in particular are associated with multidrug resistance and nosocomial outbreaks. Among the international clone lineages, A. baumannii international clone Ⅱ lineage corresponding to sequence type (ST) 2 (Pasteur scheme) or ST208 (Oxford scheme) based on multilocus sequence typing (MLST) is the most globally widespread and has also caused nosocomial outbreaks in Japan. However, MDRA species are isolated much less frequently in Japan than in other countries (see p.52 of this issue). Indeed, in Japan, MDRA species are vigilantly monitored as a drug-resistant bacteria imported from overseas (see p.53 of this issue).
Carbapenem resistance in Acinetobacter species is mainly conferred by their production of carbapenemases, including OXA-type carbapenemases and metallo-β-lactamases (see pp.51, 56 of this issue). As the above-mentioned A. baumannii international clone Ⅱ lineages are frequently OXA-type carbapenemases, OXA-type carbapenemase producers are dominant globally. However, metallo-β-lactamase producing MDRA has also been confirmed in Japan (see p.55 of this issue).
National Epidemiological Surveillance of Infectious Diseases (NESID)
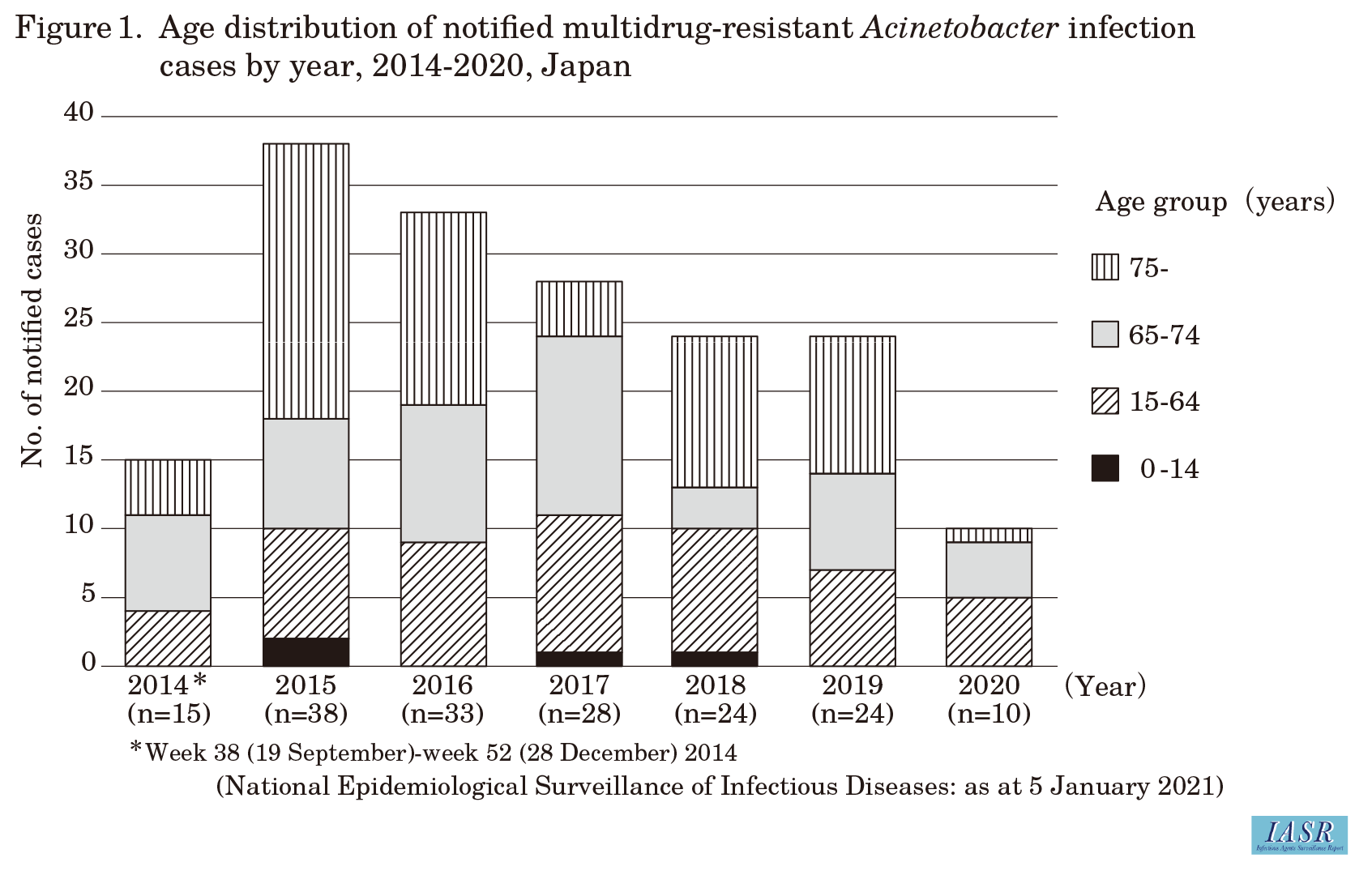
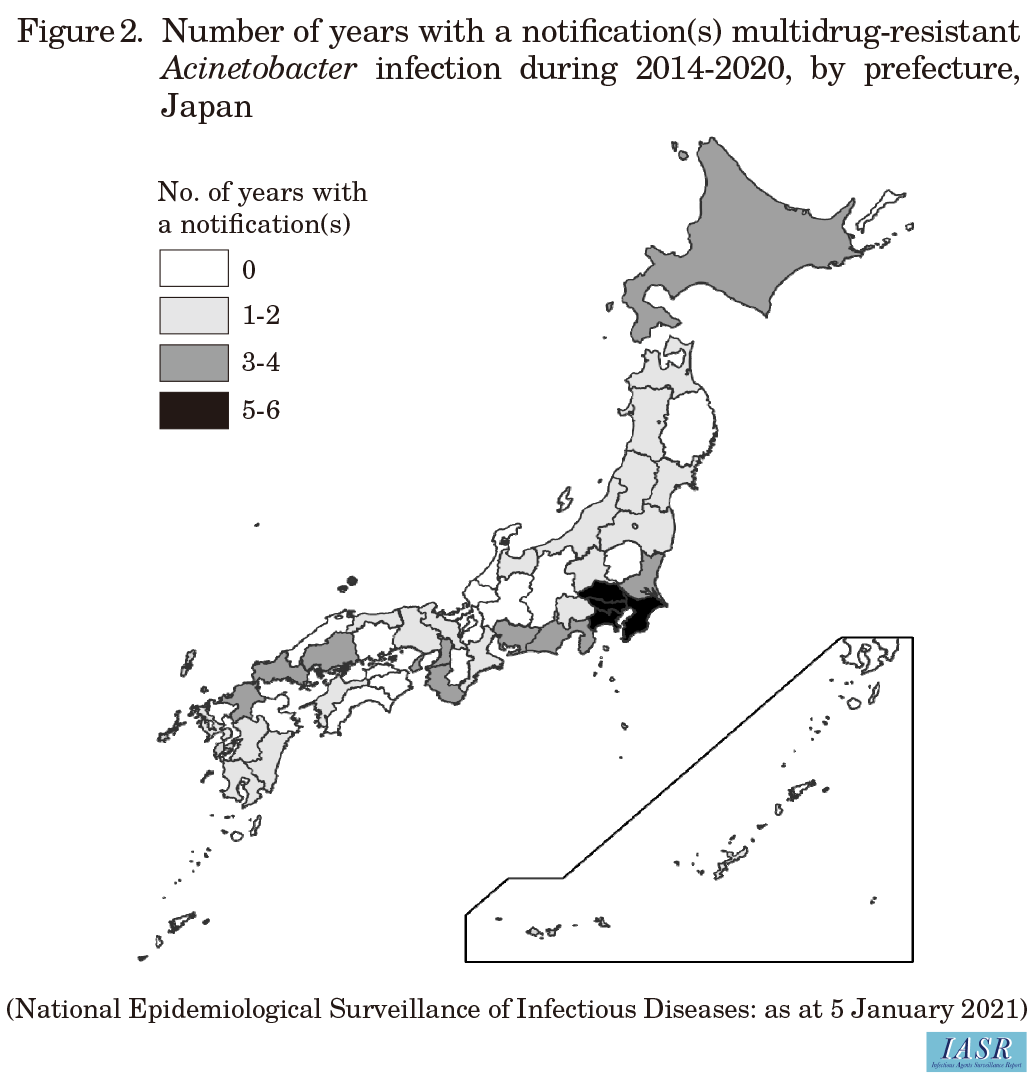
Since MDRA infections became a Category V infectious disease for notifiable disease surveillance in September 2014, there have been 172 notified cases in total (as at January 5, 2021). Of these 172 patients, 122 (71%) were male, the median age was 71 years (range: 0-97 years) at diagnosis, and 116 (67%) were aged 65 years or older. The most common type of infection (including multiple infections) was pneumonia in 92 patients (53%), followed by bacteremia/sepsis in 31 patients (18%), and urinary tract infection in 21 patients (12%). The most common type of specimen in which MDRA was detected (including detection in multiple specimen types) was sputum from 106 patients (62%), followed by blood from 21 (12%), urine from 20 (12%), and pus from 10 (6%). The number of notified cases by year revealed a decreasing trend ever since the highest number of cases was notified in 2015, when there were 38 cases; for any given year, at least half of the notified patients were 65 years or older (Fig. 1 in p.49). According to the number of notified cases by prefecture, the largest number of notifications were from Tokyo Prefecture (n = 29), followed by Saitama Prefecture (n = 27), Chiba Prefecture (n = 14), Kanagawa Prefecture (n = 14), and Hokkaido Prefecture (n = 9). Notified cases occurred intermittently for at least five years in the top four prefectures (Fig. 2), and there are concerns about MDRA becoming endemic in these areas.
 |
 |
Of the total 172 notified cases, the suspected place of infection was outside of Japan in 25 cases (15%), with Asian countries accounting for many of those cases -- China (n = 8), Vietnam (n = 4), South Korea (n = 3), Indonesia (n = 2), Thailand (n = 2), and 1 case each from India, Croatia, Peru, Malaysia, Russia, and Taiwan. Carbapenem-resistant Enterobacteriaceae infection and vancomycin-resistant enterococcal infection are also designated as Category V infectious diseases for notifiable disease surveillance; 0.4% and 1.7%, respectively, of their notifications had the place of infection being suspected as outside of Japan (based on cases notified during 2015-2018, NESID Program Annual Report), suggesting that MDRA infections are characterized by a relatively larger proportion of cases with overseas transmission.
Japan Nosocomial Infections Surveillance (JANIS), Japan Ministry of Health, Labour and Welfare
The JANIS clinical laboratory division publishes summary data on isolates that fulfill the laboratory criteria needed for notification of MDRA. These summary data differ from NESID data in that they do not distinguish between asymptomatic carrier cases and symptomatic cases, and reports are restricted to isolates from medical institutions that participate in JANIS. Among the 2,075 medical institutions included in the 2019 annual report, 29 (1.4%) institutions isolated MDRA strains from a total of 98 case-patients, and the annual number of case-patients with MDRA isolation has not increased since 2017 (see p.58 of this issue).
Treatment for MDRA and Precautions Regarding Antimicrobial Susceptibility Testing
Acinetobacter species were originally susceptible to many types of antimicrobials before resistant isolates started to emerge in Europe and the US circa the 1990s, and multidrug-resistant isolates began appearing in many countries, including in Asia, circa 2000. Multidrug-resistant isolates exhibit resistance to almost all antimicrobials used to treat infections of Gram-negative bacteria. Although colistin and tetracyclines are therapeutic options for infections of MDRA, their effectiveness and side effects are of concern, and isolates resistant to these antimicrobials have emerged. The development of novel antimicrobials, such as cefiderocol, a siderophore cephalosporin, is awaited (see p.59 of this issue).
Antimicrobial susceptibility testing devices are becoming more common in the clinical setting. It should be noted that one such device, VITEKⓇ 2 (bioMérieux), is unable to accurately determine amikacin resistance in Acinetobacter species; therefore, additional confirmation using another antimicrobial susceptibility testing method is advised when deemed necessary (see p.51 of this issue).
Conclusion
A 2017 notice (HSB / TIDCD Notification No. 0328- 4) prescribes the promotion of testing for antimicrobial resistance genes at public health institutes in the event of an MDRA infection case notification, enabling the progressive development of the pathogen surveillance system. Although the NESID system carries out surveillance for symptomatic cases of MDRA infection, both symptomatic patients and asymptomatic carriers need to be covered for infection prevention and control. The 2014 notice “Measures against nosocomial transmission in medical institutions” (HPB / MCPD Notification No. 1219-1) prescribes rigorous infection control measures, in line with outbreak response, at the point at which the first case of MDRA (including an asymptomatic carrier) is detected. In addition, any suspicion of nosocomial transmission calls for reporting to the public health center, along with the rapid implementation of appropriate measures such as seeking support from regional networks for infectious diseases prevention and control. In line with prevention measures, the notice warrants laboratory testing support from public health institutes as necessary, and strengthening of laboratory testing systems is ongoing (see p.56 of this issue). In 2018, after a report of a suspected case of nosocomial infection, another administrative communication was issued that called for rigorous prevention measures against nosocomial infections, including MDRA infections (https://www.mhlw.go.jp/content/10900000/000343986.pdf), and continued response efforts in this endeavor are required.

