Acute hepatitis of unknown aetiology in children in Japan, October 2021 – June 2022, as at 23 June, 2022
(First report)
National Institute of Infectious Diseases (NIID)
Center for Emergency Preparedness and Response
Center for Field Epidemic Intelligence, Research, and Professional Development
Center for Surveillance, Immunization and Epidemiologic Research
Since April 2022, a cluster of severe acute hepatitis cases has been reported in children under 16 years of age in Europe and the United States. Although adenovirus infection has been suspected as a potential cause, other infectious diseases and chemical substances may also cause acute hepatitis. In response to this global situation, the Japanese Ministry of Health, Labor, and Welfare (MHLW) has requested to report acute hepatitis of unknown cause in children under 16 years of age through an administrative notice.
Here, we describe reported cases collected by MHLW and the National Institute of Infectious Diseases, as well as the reports based on the existing national surveillance related to acute viral hepatitis and adenovirus infection.
Summary
- As at 23 June, 2022, a total of 62 cases of probable cases of acute hepatitis of unknown aetiology in children, which fulfilled the working case definition probable were reported. To date, no cases requiring liver transplantation or death have been reported. No specific trends were identified in the timing of case onset, geographic region of residence, or the detected pathogens.
- Related information based on the National Epidemiological Surveillance of Infectious Diseases (NESID) showed no signs of an increase in acute hepatitis or adenovirus infection in children in Japan.
- Based on this information, the number of cases in Japan is not expected to increase rapidly. The cause and etiology of these cases are still under investigation.
Overview of reported cases of acute hepatitis of unknown cause in children
The working case definitions in the survey conducted by the MHLW and NIID are as follows.
Hospitalized cases with hepatitis of unknown aetiology diagnosed since 1 October, 2021, who met any of the following criteria: (1), (2), or (3)
(1) Confirmed cases: Not applicable at present.
(2) Probable cases: A person presenting with acute hepatitis (non-hepatitis A-E) with serum transaminase > 500 IU/L (AST or ALT), who is 16 years and younger and needs to be hospitalized.
(3) Epidemiologically linked: A person presenting with acute hepatitis (non-hepatitis A-E) of any age who is in close contact with (2) .
Sixty-two probable cases meeting the above working case definition have been reported in Japan (Table 1), but no significant trend has been observed in terms of causative pathogens or the timing of disease onset.
Table 1: Incidence of hospitalized cases in Japan that meet the provisional case definition (as at 10:00, 23 June)
|
Number of probable cases |
Epidemiologically
linked cases |
Number of SARS-CoV-2 PCR test- positive cases |
Number of adenovirus PCR test-positive cases |
|
62 |
0 |
5 |
5 |
Of the 62 cases, 34 (55%) were male and 28 (45%) were female, with a median age of 5 years (interquartile range 2–10 years) (Table 2). Of the cases in which information was available, 28% (17/60) had underlying diseases (Tables 2 and 3).
The proportion of those who received at least one dose of the COVID-19 vaccine was 22% (12/55), and the proportion of those with prior SARS-CoV-2 infection to hepatitis onset prior to the onset of hepatitis was 9% (5/58) (Table 2).
Cases have been reported in all regions of Japan, with no clear regional accumulation at this time.
The diagnostic criteria for acute liver failure are "liver damage in a normal liver or a liver with normal hepatic reserve capacity, and a prothrombin time of 40% or less or an INR value of 1.5 or more based on severe hepatic dysfunction within 8 weeks after the first symptoms appear" (M W, " The Intractable Hepato-Biliary Diseases Study Group in Japan”:2015 revised edition).
Four (13%) of the 32 cases for which information on PT-INR was available met these diagnostic criteria for acute liver failure. Two of these cases had underlying diseases, and none showed regional bias (Figure 1).
The week of onset ranged from week 40, 2021, to week 22, 2022 (Figure 1), with 93% (57/61) of cases occurring after week 7, 2022 [14 February to 20 February 2022]. After week 7, 2022, cases have been continuously reported, with a median number of two cases per week (interquartile range 1-5).
The number of cases increased after the administrative notice. Week 17 of 2022, however, should be interpreted with caution, as it is possible that cases were not adequately reported because of the need for medical facilities to review their medical records.
In cases for which information was available, the clinical symptoms were as follows: fever of 37.5°C or higher in 68% (41/60 patients), gastrointestinal symptoms (those presenting with either abdominal pain, diarrhea, or vomiting/nausea) in 52% (31/60 patients), cough in 28% (17/60 patients), jaundice in 22% (13/60 patients), pale stool in 5% (3/60 patients), and impaired consciousness in 5% (3/60) (Table 2).
The testing status of these cases is not uniform. Among the 60 patients for whom information on aspartate aminotransferase (AST) and alanine aminotransferase (ALT), indicators of liver function, were available, the median [interquartile range] values were 698 IU/L [417–1,007 IU/L] and 746 IU/L [556–1,238 IU/L], respectively. The median [interquartile range] values for total bilirubin and prothrombin time-international normalized ratio (PT-INR) were 1.0 mg/dL [0.48–4.31 mg/dL] and 1.09 [0.99–1.32], respectively, in 39 and 32 patients, respectively, for whom total bilirubin and PT-INR information were available (Table 2).
Regarding pathogen testing, mainly on whole blood, serum, stool, and respiratory-derived specimens, SARS-CoV-2 was detected in 8% (5/59) of cases for which information was available. In addition, adenovirus was detected in 9% (5/58) of the 58 cases for which the results of the adenovirus test were known. One case was type 1 and one was type 2 (Table 1). Three cases tested positive at hospitals but negative at public health institutes (PHIs), and the adenovirus type was not determined. Seven other cases are currently undergoing further examination at PHIs. In addition, three cases of human herpesvirus (HHV)-6, two cases of HHV-7, two cases of EB virus, two cases of rhinovirus, and one case of norovirus were detected at the PHIs. Currently, there are no characteristic trends in the detection of these viruses. No information on liver biopsy results was available.
The median [interquartile range] time from onset to admission was 4 [2–9] days. Of the 62 patients, 53 (85%) had already been discharged (as of June 23), and the median [interquartile range] length of stay for the 51 patients for whom information was available was 9 [7–14] days.
Among the cases for which information was available, 19% (6/31) were admitted to the intensive care unit (ICU)/high care unit (HCU), and among these, three had underlying diseases.
Although there has been neither case in need of liver transplantation nor fatal cases, an additional period of observation is needed to confirm the outcome of the reported cases.
The cause and aetiology of these cases are still under investigation.
Figure 1. Cases that fulfill the working case definition in Japan (as at 23 June, 10am)
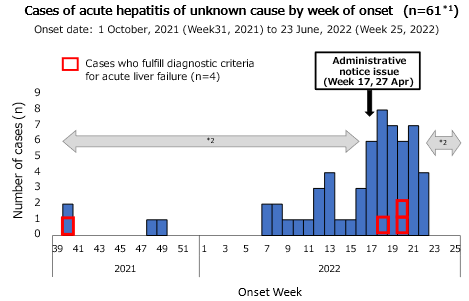
*1 Excluding a case whose date of onset was not available.
*2 The number of cases might have been affected because of the retrospective case findings, and the number of recently reported cases can be changed.
Table 2. Characteristics of hospitalized cases who fulfill the working case definition in Japan (n=62, as at 23 June, 10 am)
|
Age |
Median |
[Interquartile range] |
|
|
5 years old |
[2-10 years old] |
|
Gender |
Number of cases |
(%)*1 |
|
Male |
34 |
(55) |
|
Female |
28 |
(45) |
|
Underlying medical diseases |
Number of cases |
(%)*1 |
|
17 |
(28) |
|
|
COVID-19 immunization history |
Number of cases |
(%)*1 |
|
At least one dose |
12 |
(22) |
|
History of COVID-19 prior to onset of disease |
Number of cases |
(%)*1 |
|
Yes |
5 |
(9) |
|
Clinical symptoms*2 |
Number of cases |
(%)*1 |
|
Fever of 37.5°C or higher |
41 |
(68) |
|
Gastrointestinal symptoms *2 |
31 |
(52) |
|
Cough |
17 |
(28) |
|
Jaundice |
13 |
(22) |
|
White stools |
3 |
(5) |
|
Impaired consciousness |
3 |
(5) |
|
Test results*4, * |
Median |
[Interquartile range] |
|
AST (IU/L) |
698 |
[417-1,007] |
|
ALT (IU/L) |
746 |
[556-1,238] |
|
|
Median |
[Interquartile range] |
|
Total bilirubin (mg/dL) |
1.0 |
[0.48-4.31] |
|
PT-INR |
1.09 |
[0.99-1.32] |
|
Outcome |
Number of cases |
(%)*1 |
|
ICU/HCU admission |
6 |
(19) |
|
Liver transplantation |
0 |
(0) |
|
Death |
0 |
(0) |
*1 Denominators include cases with available data.
*2 Overlapped data are included
*3Persons presenting with abdominal pain, diarrhea, or vomiting/nausea
*4 AST, ALT, total bilirubin, and PT-INR were the maximum values up to the time of reporting.
*5AST, ALT, total bilirubin, and PT-INR were based on information obtained from 60, 60, 39, and 32 cases, respectively.
Table 3. Classification of underlying diseases (n=17, as at 10:00, 23 June)
|
Underlying diseases *1 |
Number of cases |
|
Syndromes involving changes in chromosomes or genes*2 |
3 |
|
Psychomotor retardation |
3 |
|
Congenital heart disease |
2 |
|
Very low birth weight*2 |
2 |
|
Endocrine disorder |
2 |
|
Autoimmune and collagen diseases |
2 |
|
Congenital metabolic disorder |
1 |
|
Primary immunodeficiency syndrome |
1 |
|
Other |
3 |
*1 Overlapped data are included
*2 Among these, two patients fulfilled the diagnostic criteria for acute liver failure mentioned above.
Relevant information based on the National Epidemiological Surveillance of Infectious Diseases system
〇 National Epidemiological Surveillance of Infectious Diseases (NESID)
- There are no signs of an increase in the number of reported pediatric cases of "viral hepatitis (excluding hepatitis E and hepatitis A)" (as at 2 June ).
According to the National Epidemiological Surveillance of Infectious Diseases (NESID) system under the Infectious Diseases Control Law, viral hepatitis (excluding hepatitis E and hepatitis A) is designated as a category V infectious disease, and a physician who diagnoses the disease is required to notify the Public Health Center within seven days. The number of reported cases was lower in 2020-2021 than in 2017-2019, and there was no increase in the number of reported cases in weeks 1-21 in 2022. Pediatric reports are rare, with only three cases reported in children aged 16 years or younger since 2021. Since 2017, hepatitis B and hepatitis C have consistently been the most frequent types, accounting for at least 70% of cases (no case of hepatitis D has been reported). Since 2021, the number of reported viral hepatitis cases other than hepatitis B, C, or D has increased slightly, but cytomegalovirus and EB virus have been responsible for the majority of cases. One case of adenovirus type 5 was reported during this period; however, this was not a pediatric case.
- There are no signs of a major epidemic of syndromes associated with adenovirus (as at 15 June).
Syndromes associated with adenovirus infection include infectious gastroenteritis, pharyngoconjunctival fever, and epidemic keratoconjunctivitis. Under the NESID system, these three diseases are reported under sentinel surveillance (category V infectious diseases), and the designated sentinel facility must report the aggregate number of cases to the Public Health Center on a weekly basis (approximately 3,000 pediatric sentinel sites nationwide for infectious gastroenteritis and pharyngoconjunctival fever and approximately 700 ophthalmology sentinel sites nationwide for epidemic keratoconjunctivitis). In terms of magnitude (level), the number of reported cases per sentinel site during weeks 1-23 in 2022 was similar to or lower than those in 2017-2019. Furthermore, there are no unusual trends in 2022 compared to those from 2017-2019.
- Based on reports to the NESID’s Infectious Agents Surveillance System (laboratory-based surveillance), there are no signs of high-level circulation of adenovirus* (as at 3 June [as at 16 June for part of the data]).
According to information on pathogenic agents reported by the Public Health Institutes to the laboratory-based surveillance system (information on pathogens detected in specimens collected at sentinel sites, other medical facilities, and Public Health Centers), there is no indication that the number of adenovirus reports is increasing or at high levels in 2022.
In addition, the number of adenovirus reports in laboratory-based surveillance in 2022 did not increase and remained at a low level (as at 16 June), even when restricted to pathogens detected in cases of gastroenteritis symptoms (diarrhea, nausea/vomiting, and abdominal pain) reported from pediatric sentinel sites. Data from the NESID system showed an increase in the number of infectious gastroenteritis cases per pediatric sentinel site from the end of 2021 to early 2022 (to a level similar to that during the same period–2017-2019). However, norovirus was detected in the majority of cases reported with gastroenteritis symptoms from pediatric sentinel sites to laboratory-based surveillance during this period, and adenovirus detection was few. Adenovirus detection has remained low since April 2020 and continues to be lower than that of the same period in 2017-2019.
Through laboratory-based surveillance, four cases were reported in which "hepatitis" was recorded with adenovirus detected from the specimens from January 2017 to April 2022 (three cases in 2017 and one case in 2019). These cases were all less than 3 years of age, and the adenovirus type was type 1, type 5, or type 6. In addition, there have been a few cases reported each year, the majority of which involved children, where "liver dysfunction" was noted with adenovirus being detected from the specimen. To date, four cases have been reported in 2022, which is similar to the number reported in previous years.
*In laboratory-based surveillance, as there is no stipulation regarding the number of days between detection and reporting, reporting may be delayed; thus, care in interpretation is necessary, especially for the most recent information.
〇 At present, there is no evidence of an increase in severe hepatitis or transplantation cases in children in the physician networks of academic societies and other medical institutions that perform pediatric liver transplantation.
We express our sincere gratitude to all medical institutions, public health centers, local government offices, and public health institutes throughout Japan for their cooperation.
Reference Data
- Infectious Disease Surveillance in Japan (as of February 2018)
- National Institute of Infectious Diseases, Infectious Diseases Weekly Report (IDWR)
- National Institute of Infectious Diseases, Infectious Agents Surveillance Report (IASR)